What’s already known?
The herbal compound, Curcumin, is rich in anti-inflammatory and antioxidant properties¹ and has been found in numerous clinical studies to induce remission in IBD patients and promote long-lasting remission maintenance².
What’s new from this study?
A gut-directed formulation of Curcumin has been found to vastly improve its effects on intestinal inflammation in IBD specifically in mild-moderate disease activity. It is a safe add-on therapy to standard treatment for improving outcomes.
A unique form of Qing Dai has been confirmed as a highly effective and safe treatment that induces rapid remission and symptom relief for IBD in placebo-controlled trials even in moderate-severe cases who are not responding to biologics or immune-suppressant medications.
What are the implications for the future in IBD?
We now have an evidence-based protocol for the correct use, dosage, and treatment duration of Qing Dai and curcumin, enabling the safe, effective integration of this combination treatment – CurQD®.
This program allows for the tailored administration of CurQD® with careful tapering of Qing Dai after induction and substitution with gut-directed curcumin to improve safety, patient outcomes, mucosal healing, and long-term remission in patients with IBD, including those with active disease who did not previously respond to standard medication.
How does it work?
CurQD® contains unique molecules, Indigo and Indirubin³ (constituents of QD), which exert an agonistic effect on the aryl hydrocarbon receptor (AhR) pathway⁴.
Aryl hydrocarbon receptors line the gastrointestinal tract, and when activated, send signals to combat chronic inflammation, heal mucosal damage, and restore homeostasis to the intestinal immune system. The molecules within CurQD® activate these receptors, accelerating the reconstitution of intestinal integrity and contributing to mucosal healing and induction in IBD patients.
The AhR pathway is a new focus of emerging treatments for IBD. CurQD® is the first therapy shown in clinical trials to induce remission by activating this pathway.
This multi-center trial used a combination of curcumin with Qing Dai (CurQD®) on UC patients who had all failed at least one line of pharmaceutical UC treatments for 8 weeks, with a rollover to an additional 8 weeks maintenance treatment for responders.
- 97.5% of patients had previously not responded to 5ASA
- 48.8% of patients had previously not responded to biologics and/or immunomodulators experienced.
- Nearly one-third of patients had previously not responded to immune suppressant therapy
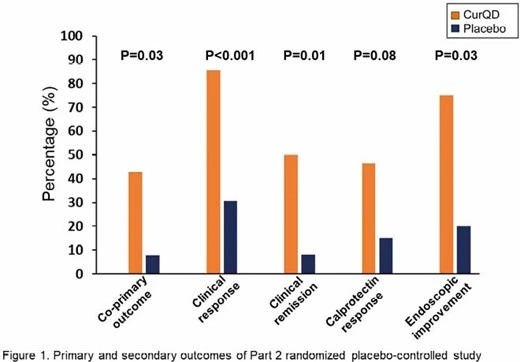
At week 8, 50% of patients achieved clinical remission, and 85.7% achieved clinical response.
Among those who responded to CurQD®, an additional 8 weeks of treatment with curcumin alone resulted in:
- 93% with maintained clinical response
- 80% with significant reduction in calprotectin
CurQD® was found greatly superior to placebo for induction of response and remission in active UC patients, including in patients with moderate-severely active UC, of whom half were biologics or immuno-modulators experienced.
A limited CurQD® induction phase followed by curcumin maintenance appeared to maximize the efficacy and safety of this combination therapy.
This multi-center retrospective study observed 88 UC patients from 5 medical centers who all had active disease at the time of treatment initiation.
- 82.9% had previously not responded to corticosteroids.
- 43.2% had previously not responded to at least one biologic/small molecule
- 28.4% had previously not responded to over 2 biologics/small molecule
Within 12 weeks:
- 70.4% of patients showed clinical response.
- 51.1% of patients showed clinical remission.
Among biologic/small molecules-experienced patients, 72.3% showed a clinical response, and 44.7% achieved clinical remission.
Of the patients on corticosteroids at baseline, 26.9% achieved corticosteroid-free remission. Of the patients on corticosteroids at baseline, 50% were weaned off corticosteroids by the end of induction.
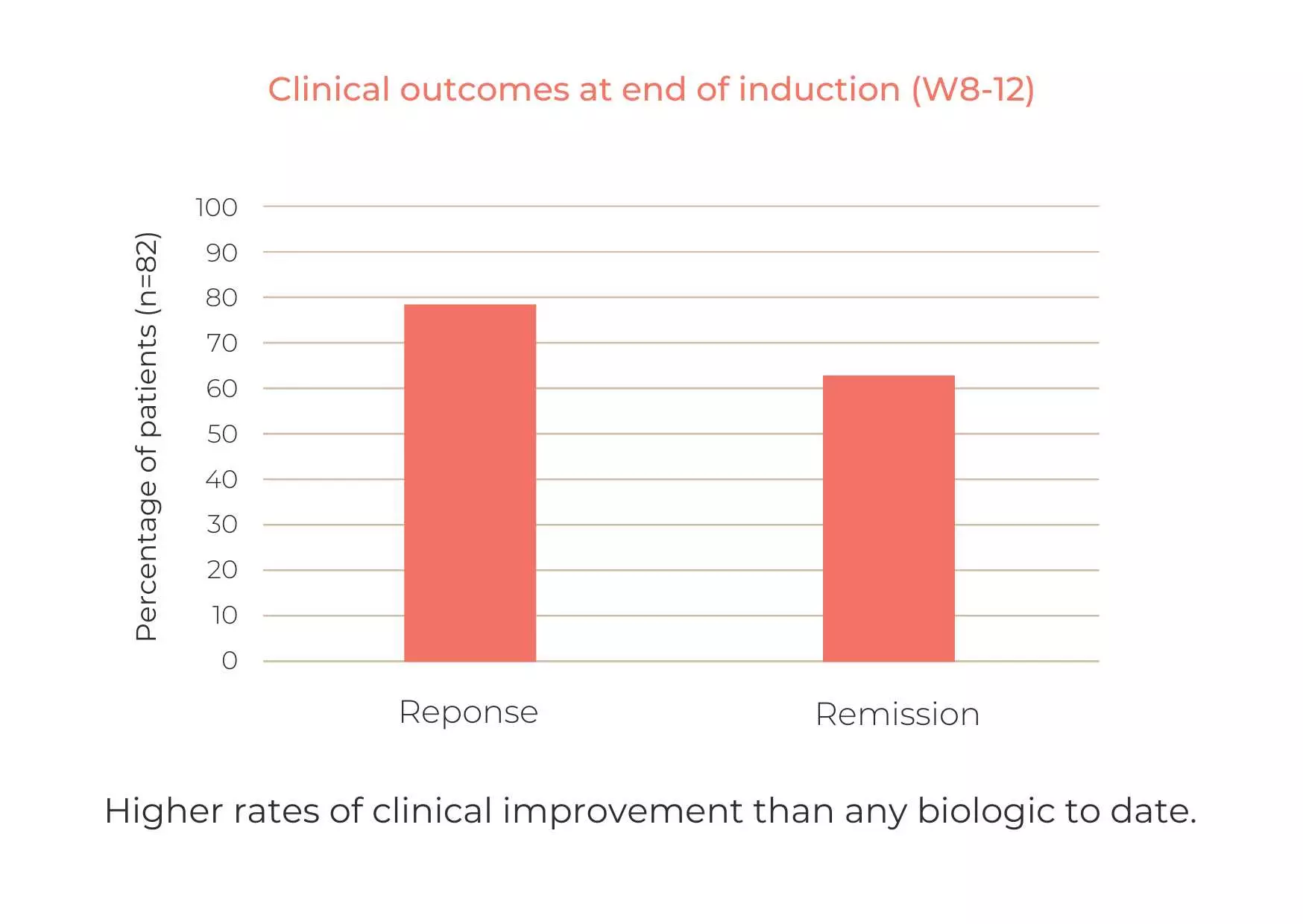
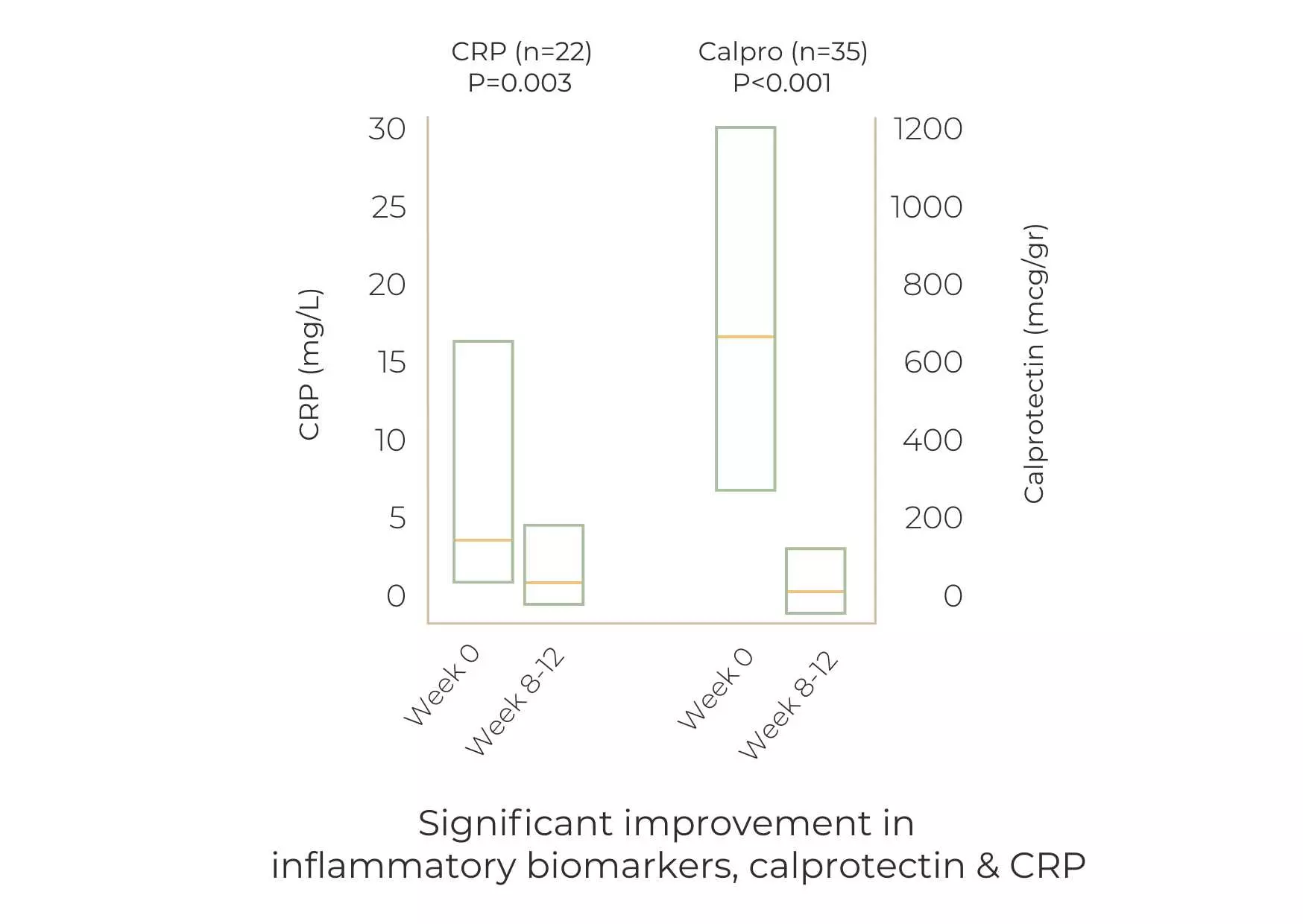
The study shows that a combination of curcumin and Qing Dai (CurQD®) is effective for inducing response and remission in patients with active UC, including patients who did not respond to biologic or small molecule therapy.
Curcumin-QingDai (CurQD) combination for moderate-severe ulcerative colitis: A report of two cases
In a case report on two UC patients, CurQD® treatment led to complete remission, mucosal healing, and long-term maintenance. These were some of the first patients treated with the combination of Qing Dai and Curcumin, and both remain in remission to this day, roughly 5 years later, using only CurQD® and no other medication.
Patient 1 was a 24-year-old with extensive, severe UC for 2 years, which entailed prolonged hospitalization. Although he improved with infliximab, he still suffered severe symptoms, including bleeding. 10 days into CurQD® treatment, bleeding ceased and the patient achieved complete remission within several weeks. He has since remained in complete remission on CurQD®, over 31 months later. No adverse events were noted.
Patient 2 was 56 years old with extensive UC for a year. She did not respond to mesalamine, nor did her symptoms improve with budesonide-MMX (including bleeding, weakness, and abdominal pain). After starting CurQD®, the patient’s symptoms resolved rapidly. 5 months later, she showed complete mucosal healing. She has since maintained remission with a tapered dose of QD.
This clinical experience shows that CurQD® is effective in moderate-severe UC patients, including those resistant to biologics and/or corticosteroids.
References
- Peng Y, Ao M, Dong B, et al. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des Devel Ther. 2021;15:4503-4525. Published 2021 Nov 2. doi:10.2147/DDDT.S327378
- Lin Y, Liu H, Bu L, Chen C, Ye X. Review of the Effects and Mechanism of Curcumin in the Treatment of Inflammatory Bowel Disease. Front Pharmacol. 2022;13:908077. Published 2022 Jun 20. doi:10.3389/fphar.2022.908077
- Chia-Lin Lee, Chien-Ming Wang, Yueh-Hsiung Kuo, Hung-Rong Yen, Ying-Chyi Song, Yu-Lun Chou, Chao-Jung Chen, IL-17A inhibitions of indole alkaloids from traditional Chinese medicine Qing Dai, Journal of Ethnopharmacology, Volume 255, 2020, 112772, ISSN 0378-8741, doi.org/10.1016/j.jep.2020.112772.
- Hubbard TD, Murray IA, Perdew GH. Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation. Drug Metab Dispos. 2015;43(10):1522-1535. doi:10.1124/dmd.115.064246
